Over 7,000 research studies worldwide use InBody
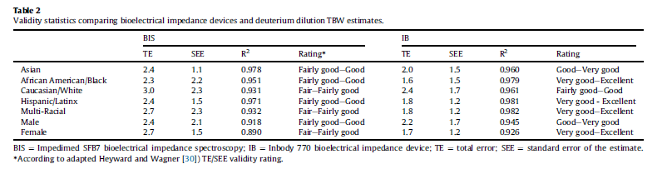
A study with 109 multi-ethnic participants looked at comparisons between bioelectrical impedance spectroscopy (BIS) devices, including the InBody (IB) 770, and deuterium dilution for measures of total body water (TBW). For baseline measures of TBW, each participant provided a saliva sample and completed BIS and IB measurements of TBW, followed by ingestion of deuterium oxide (D2O). Participants were asked to provide a second saliva sample for TBW analysis after a period of equilibration. Researchers found that “IB estimates were excellent to very good (TE = 1.95 L, R2 = 0.975)”. They further stated that “Validity results did not vary meaningfully between race and ethnicity.”
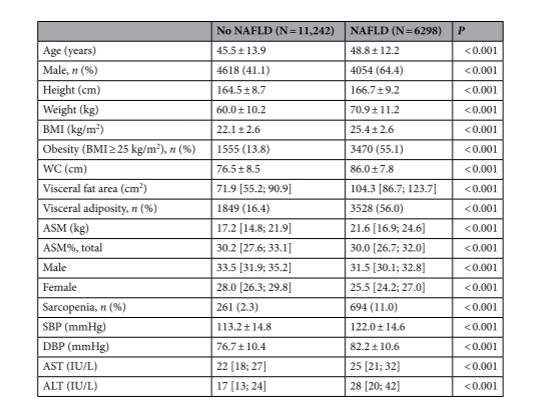
In a 2023 study published by Scientific Reports assessing the effects of interactions between visceral adiposity, obesity, and sarcopenia on NAFLD, the accuracy of the InBody 720 was compared against computed tomography (CT) in 1,927 subjects. The authors found a “significant strong correlation between measurements with the InBody 720 and those measured with CT (ASM, r=0.898, P<0.001 and VFA, r=0.714, P<0.001)”. The study concluded that positive associations, and additive interaction effects, were shown between NAFLD and sarcopenia, obesity, and visceral adiposity.
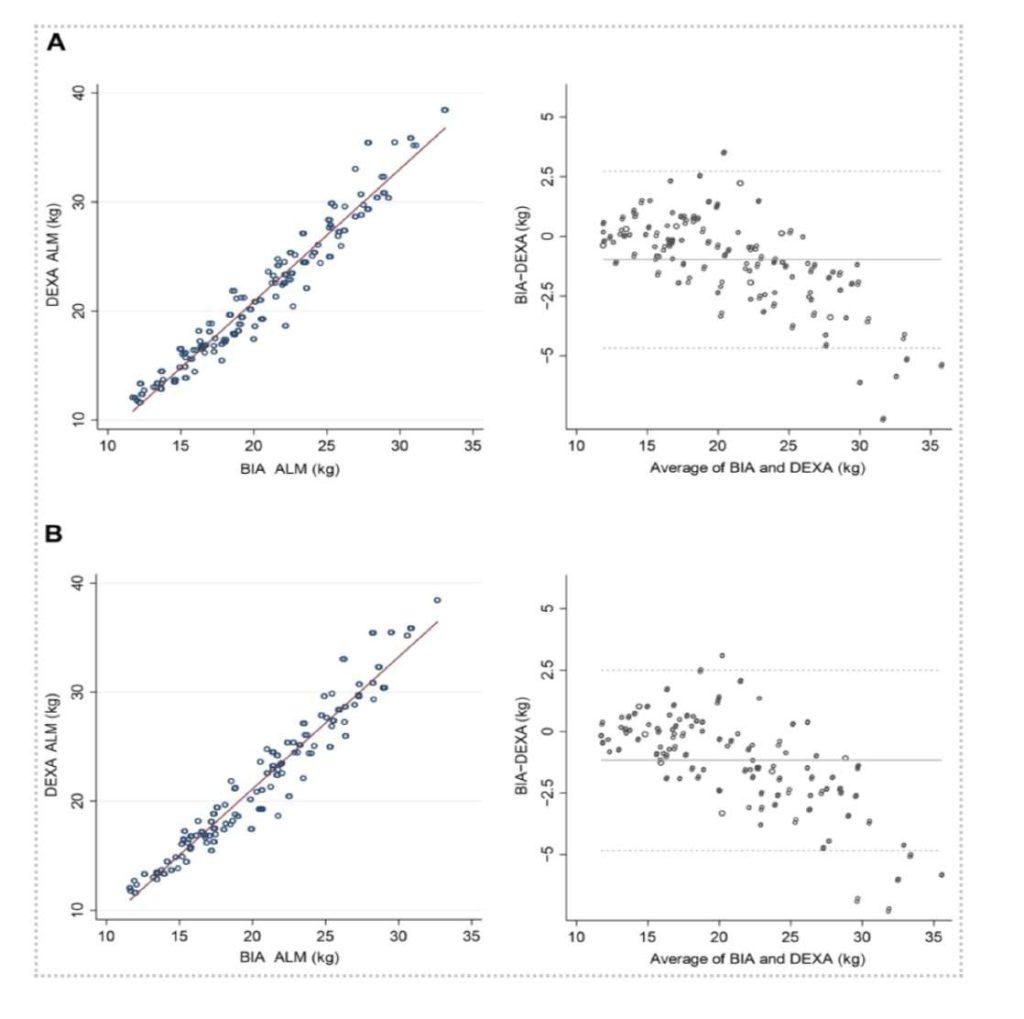
Now with more frequencies, and frequencies up to 3000 kHz, the InBody 970 and the BWA 2.0 devices provide not only more outputs, but greater precision. Life journal in a July 2022 publication evaluated the newest InBodydevices compared to dual-energy X-ray absorptiometry (DEXA) in a cohort of 109 individuals. What they found was that high-frequency InBody devices showed a “high correlation with the DEXA results” for assessingappendicular lean mass (β ≥ 0.95), fat-free mass (R2 ≥ 0.95), and percent body fat (R2≥ 0.89).
Yi, Y.; Baek, J.; Lee, E.; Jung, H.; Jang, I. (July 2022). “A Comparative Study of High-Frequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Body Composition”. Life 2022, 12(7), 994; https://doi.org/10.3390/life12070994
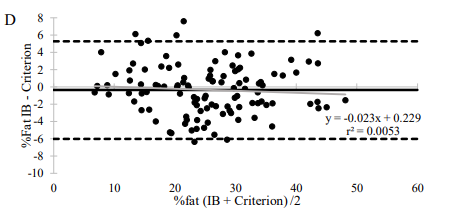
A 2022 study published by the British Journal of Nutrition compared the accuracy of DSM-BIA (InBody 770) amongst body composition gold standards (DXA, ADP) and BIS with 110 participants of varying races and ethnicities. The study found that alongside DXA and ADP, InBody provided “excellent to ideal” measures of percent body fat and “ideal” fat free mass measurementscompared to the criterion. Moreover, our devices can be used in a multi-ethnic sample “to obtain highly valid results” without estimations based on ethnicity.
The Journal of Pediatric Gastroenterology and Nutrition published a 2020 study that evaluated the accuracy of different BIA devices relative to DXA in children with obesity. Amongst other BIA, InBody stood out as it was “relatively accurate for estimating body fat percentage” and was not limited to patients younger than 10 or patients with high body fat percentage (>50%). Beyond being a “reasonably accurate alternative to DXA” in pediatric obesity, InBody is available at a tenth of the cost.
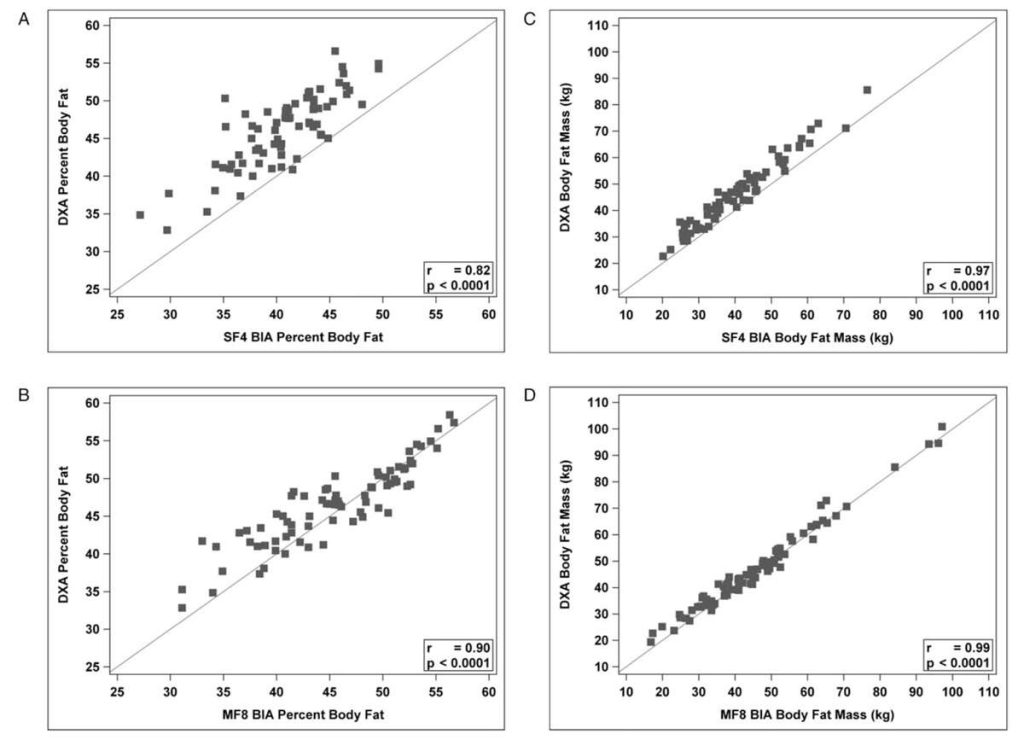
Researchers from the Mayo Clinic compared InBody technology to DXA, a body composition gold standard, for estimating fat-free mass and percent body fat in a 2020 study. They validated InBody as 98% correlated to DXAand overall had good agreement amongst all BMI categories. The authors note that InBody “allows providers to further risk stratify patients and develop treatment plans.” On another note, InBody “takes the same amount of space as a scale and allows our clinical assistants… to obtain body composition measurements in a few minutes while obtaining other vitals (eg, weight, heartrate, and blood pressure).”
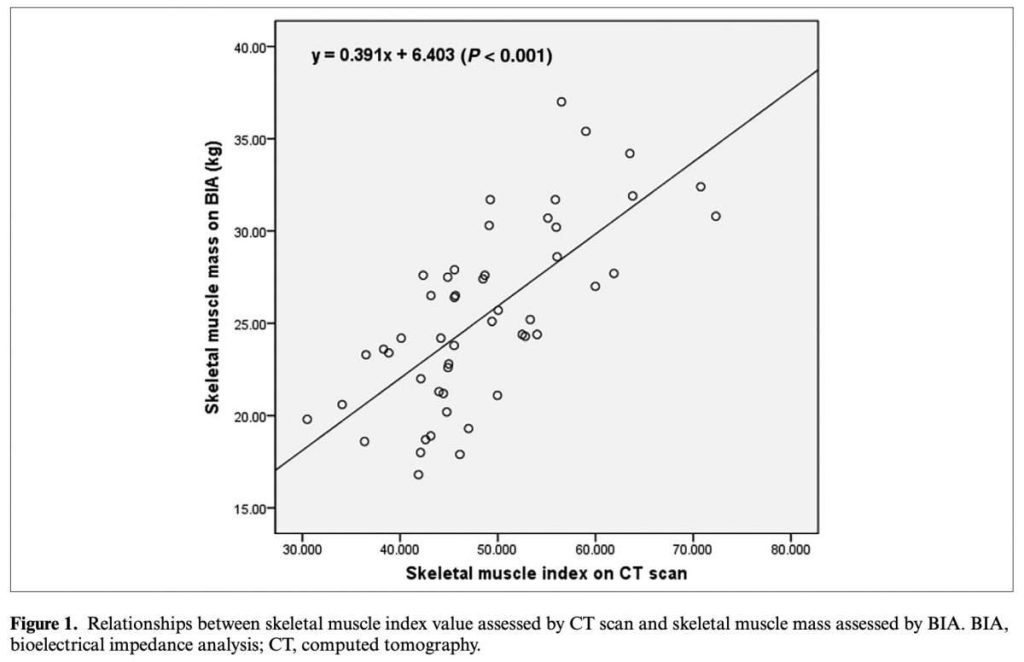
A 2020 publication from Nutrition in Clinical Practice evaluated the accuracy and reliability of InBody compared to computed tomography (CT) to assess skeletal muscle mass (SMM) in colorectal cancer patients. In their results, they found that InBody SMM has “very significant correlation” (p<0.001) to CTeven after adjusting for age, weight, and BMI (p<0.001). Overall, the authors suggest that InBody could be “an alternative to CT scan for the assessment of SMM in colorectal cancer patients.”
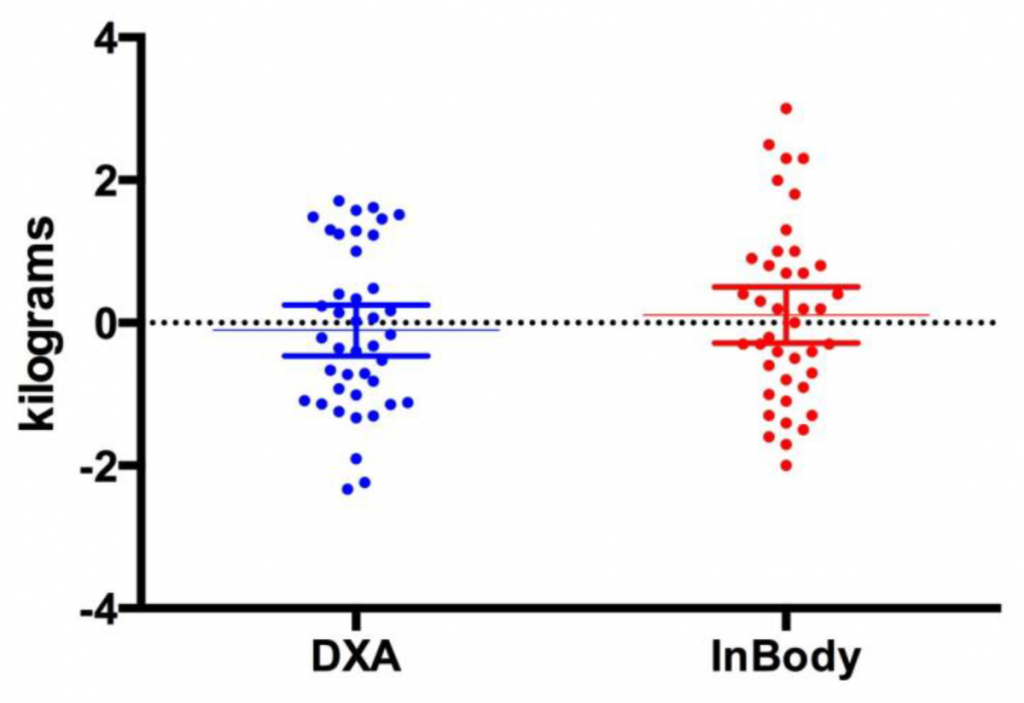
A 2019 study from the Journal of Functional Morphology and Kinesiology compared InBody 770 and DXA for body composition changes in exercise-trained men and women after a 4-week program. The researchers found that the device “can predict changes in body composition (i.e., fat mass, fat-free mass, and percent body fat) similar to a DXA.” InBody’s multiple frequencies “may be less subject to error” and “likely a superior method for the estimation of total body composition.”
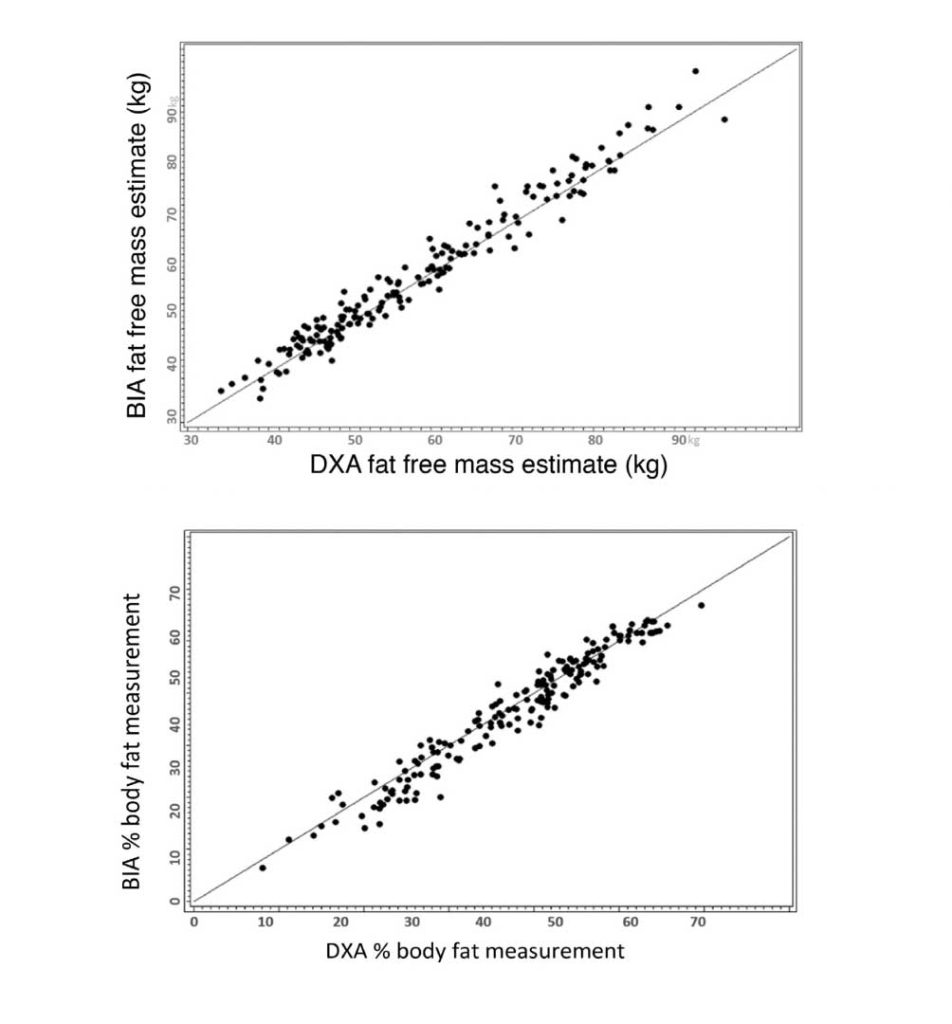
Researchers in the American Journal of Clinical Nutrition assessed the integration of DXA and InBody for a rapid body composition model in a 2018 clinical population study. What they found was that InBody had “high agreement” with D20 TBW measurements, the gold standard for assessing body water. Nonetheless, InBody is “an appealing method for clinical TBW measurement owing to its low cost, rapid results, and amenability to field calibration.”

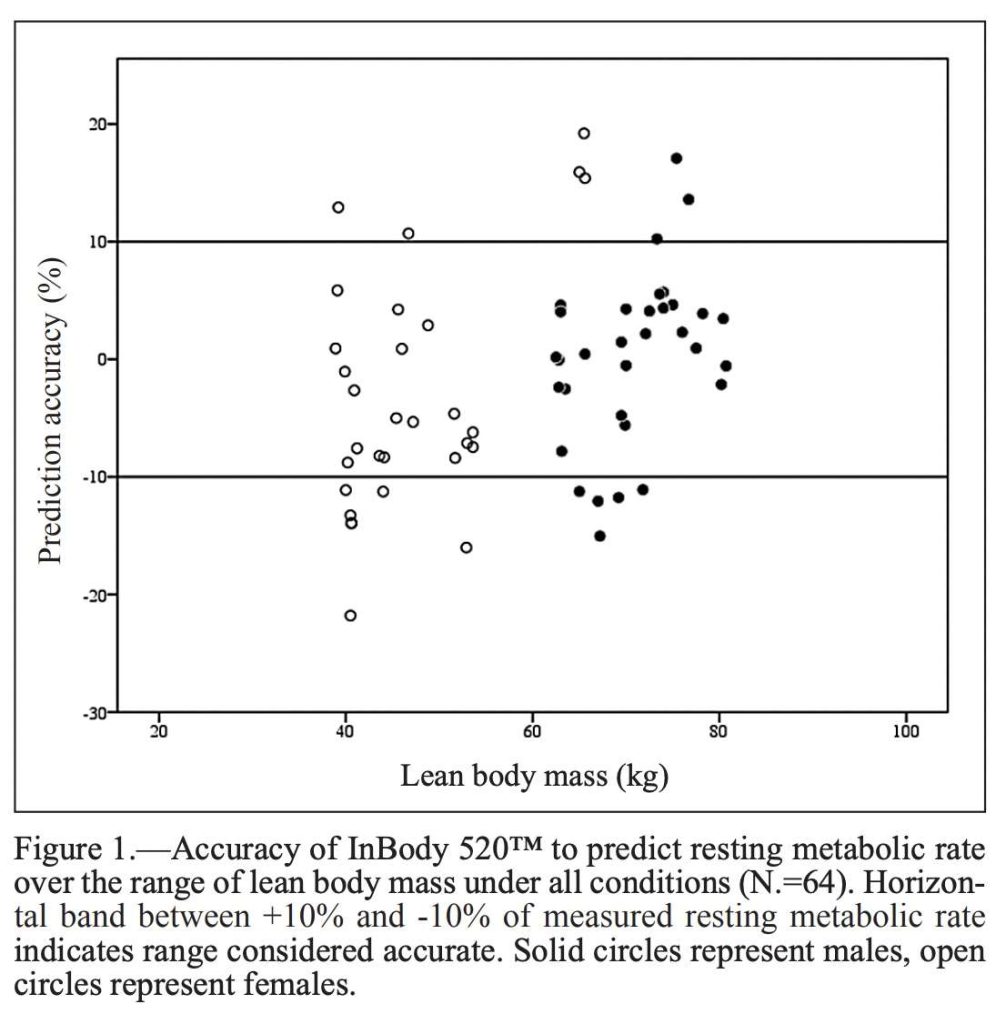
In a 2018 study published by the Journal of Sports Medicine and Physical Fitness, a team investigated the validity in an InBody device for predicting resting metabolic rate (RMR). The team found that InBody “provides valid measurements of RMR” when compared to gas analysis, the current standard. RMR values based on the InBody analysis was “a valid, more practical, and less time-intensive alternative to determine RMR in a clinical setting.”
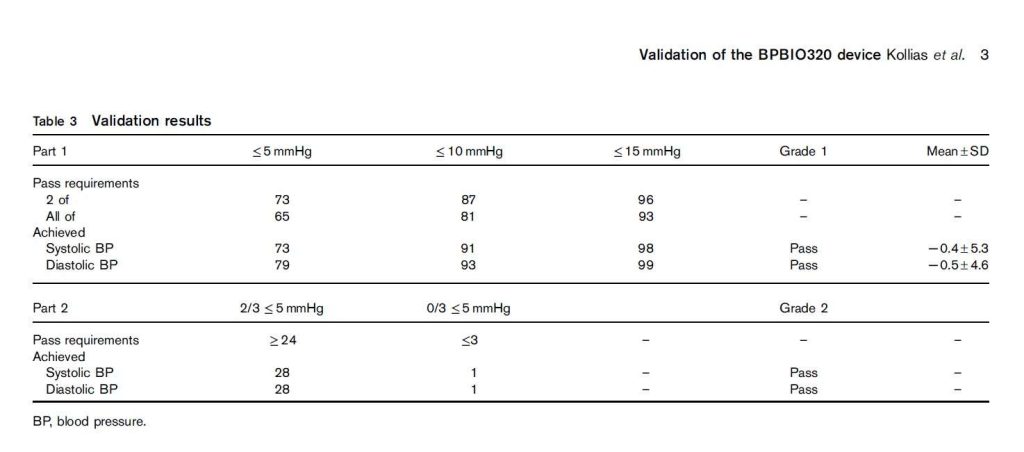
“The single-cuff oscillometric device InBody BPBIO320 developed for self-measurement by adults in public spaces passed all the validation requirements of the 2010 ESH-IP and can be recommended for clinical use.” The test–reference BP difference was 0.5±4.3/−1.1±4.3mmHg (systolic/diastolic) in participants with arm circumference less than 31.4 cm (n=17) and −1.3±6.0/0.2±4.8mmHg in those with an arm circumference of at least 31.4 cm (n=16). This indicates that the margin of error is ~5/5.5 mmHg (systolic/diastolic) in those with smaller arms, and ~7/5 mmHg (systolic/diastolic) in those with higher arm circumference.
Kollias, A., Stambolliu, E., Kyriakoulis, K. G., Papadatos, S. S., & Stergiou, G. S. (2019). Validation of the single-cuff oscillometric blood pressure monitor InBody BPBIO320 for public use according to the 2010 European Society of Hypertension International Protocol. Blood pressure monitoring, 24(1), 30-32.
InBody BWA is a global leader in body composition technology, dedicated to improving lives by enhancing the standard of care for critically ill patients. With our advanced body water analysis, clinicians and researchers can deliver more precise, effective patient care.
2550 Eisenhower Ave,
Suite C-209
Trooper, PA 19403
Copyright© 2024 by InBody BWA Inc. All rights reserved.